Kind of bond in chemistry crossword – The world of chemistry is filled with fascinating concepts, and the types of chemical bonds that hold atoms together are no exception. In this comprehensive guide, we delve into the captivating realm of chemical bonding, exploring the fundamental principles, different types of bonds, and their applications in various fields.
Join us as we embark on a journey to unravel the secrets of chemical bonds, empowering you to conquer any crossword puzzle that comes your way.
From the fundamental concepts of covalent, ionic, and metallic bonds to the more complex aspects of hydrogen bonding, resonance, and molecular orbital theory, we provide a detailed examination of each type of bond. Along the way, we uncover the factors that influence bond length and strength, the significance of bond polarity and electronegativity, and the practical applications of chemical bonding in medicine, materials science, and beyond.
Definition of Chemical Bond: Kind Of Bond In Chemistry Crossword
A chemical bond is a force that holds atoms together to form molecules or crystals. Chemical bonds are essential for the formation of matter and are responsible for the properties of substances.
There are several types of chemical bonds, including covalent bonds, ionic bonds, metallic bonds, and hydrogen bonds.
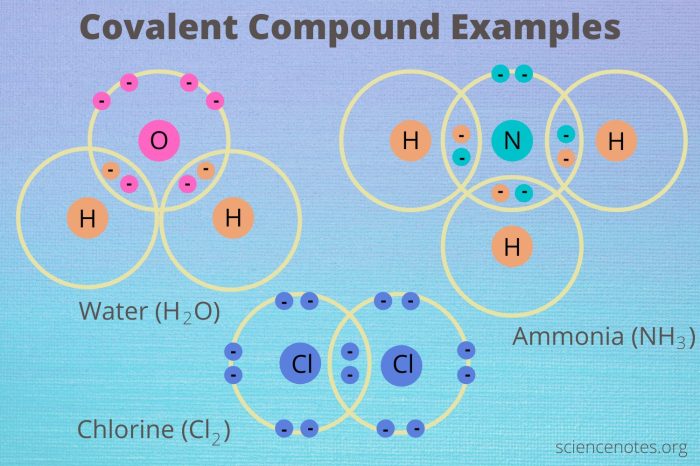
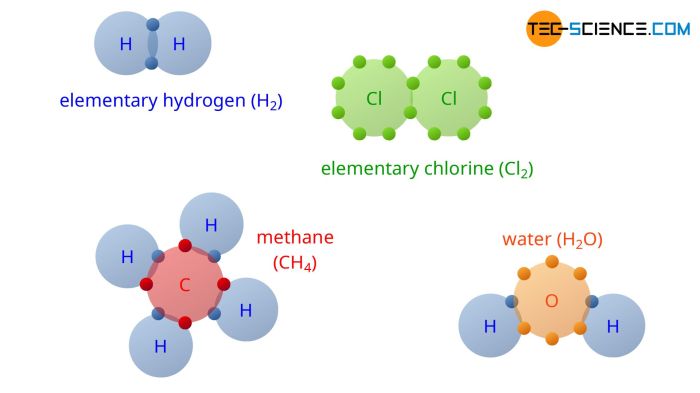
Covalent Bond
A covalent bond is a chemical bond that is formed by the sharing of electrons between atoms. Covalent bonds are the strongest type of chemical bond and are found in most organic molecules.
- Covalent bonds are formed when atoms share one or more pairs of electrons.
- The number of shared electrons determines the strength of the covalent bond.
- Covalent bonds are typically formed between atoms of nonmetals.
Ionic Bond
An ionic bond is a chemical bond that is formed by the transfer of electrons from one atom to another. Ionic bonds are typically formed between atoms of metals and nonmetals.
- Ionic bonds are formed when one atom gives up one or more electrons to another atom.
- The atom that gives up electrons becomes a positively charged ion, and the atom that receives electrons becomes a negatively charged ion.
- Ionic bonds are typically strong and are found in many inorganic compounds.
Metallic Bond, Kind of bond in chemistry crossword
A metallic bond is a chemical bond that is formed by the sharing of electrons between metal atoms. Metallic bonds are typically strong and are found in all metals.
- Metallic bonds are formed when metal atoms share their valence electrons.
- The valence electrons are free to move around the metal atom, forming a “sea of electrons.”
- Metallic bonds are responsible for the high electrical and thermal conductivity of metals.
Hydrogen Bond
A hydrogen bond is a weak chemical bond that is formed between a hydrogen atom and an electronegative atom, such as oxygen, nitrogen, or fluorine.
- Hydrogen bonds are formed when a hydrogen atom is bonded to an electronegative atom and is also attracted to another electronegative atom.
- Hydrogen bonds are weaker than covalent bonds, but they can play an important role in the structure and properties of many substances.
- Hydrogen bonds are found in many biological molecules, such as DNA and proteins.
Frequently Asked Questions
What is the strongest type of chemical bond?
Covalent bonds are generally the strongest type of chemical bond.
What is the difference between a polar and nonpolar bond?
A polar bond occurs when electrons are unequally shared between atoms, while a nonpolar bond occurs when electrons are shared equally.
What is resonance in chemistry?
Resonance is a phenomenon in which a molecule can be represented by several different Lewis structures, indicating that the electrons are delocalized over several atoms.