Compare and contrast serine proteases and aspartic proteases. – Delving into the realm of proteolytic enzymes, we embark on a journey to compare and contrast serine proteases and aspartic proteases, two prominent enzyme families that play crucial roles in diverse biological processes. This comprehensive overview unravels their structural distinctions, catalytic mechanisms, substrate specificities, physiological functions, and regulatory mechanisms, providing a profound understanding of their significance in maintaining cellular homeostasis.
Serine proteases and aspartic proteases, despite sharing the common function of proteolysis, exhibit distinct characteristics that shape their roles in biological systems. Their structural differences, including variations in amino acid sequences and key structural features, influence their catalytic mechanisms and substrate specificities.
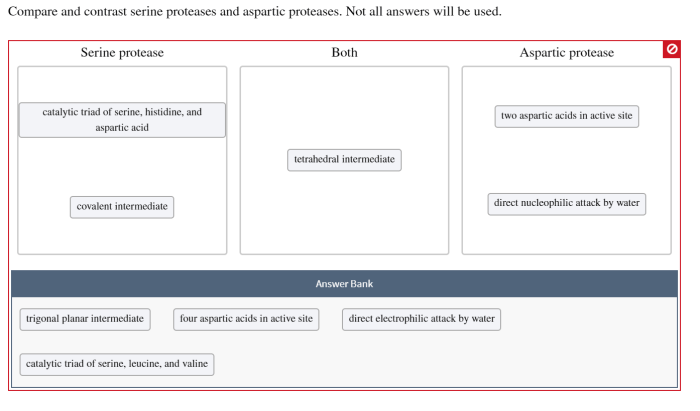
The catalytic triad in serine proteases and the catalytic dyad in aspartic proteases orchestrate the hydrolysis of peptide bonds, albeit through distinct pathways.
Serine Proteases and Aspartic Proteases: A Comparison and Contrast: Compare And Contrast Serine Proteases And Aspartic Proteases.
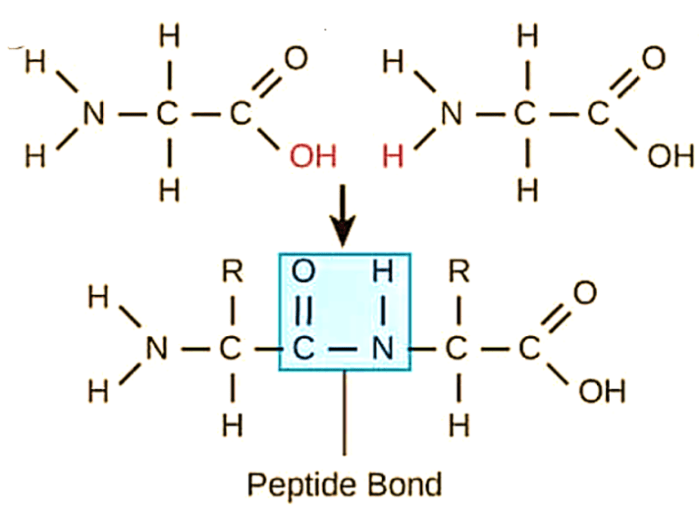
Serine proteases and aspartic proteases are two important classes of enzymes that play crucial roles in a wide range of biological processes. Both enzymes catalyze the hydrolysis of peptide bonds, but they differ in their structural features, catalytic mechanisms, and physiological functions.
Structural Differences, Compare and contrast serine proteases and aspartic proteases.
Serine proteases and aspartic proteases have distinct amino acid sequences and structural features. Serine proteases are characterized by a conserved catalytic triad consisting of serine, histidine, and aspartate residues, while aspartic proteases contain a catalytic dyad of two aspartate residues.
The catalytic triad of serine proteases forms a hydrogen-bonding network that facilitates the activation of the serine residue for nucleophilic attack on the peptide bond. In contrast, the catalytic dyad of aspartic proteases acts as a proton donor and acceptor, facilitating the hydrolysis of the peptide bond.
Catalytic Mechanisms
The catalytic mechanisms of serine proteases and aspartic proteases involve different steps and intermediates.
In serine proteases, the serine residue attacks the carbonyl carbon of the peptide bond, forming a tetrahedral intermediate. The histidine residue then donates a proton to the leaving group, facilitating the release of the cleaved peptide fragment. The aspartate residue stabilizes the transition state by interacting with the positively charged histidine residue.
In aspartic proteases, the two aspartate residues activate a water molecule, which then attacks the carbonyl carbon of the peptide bond. The resulting tetrahedral intermediate is stabilized by the positively charged side chains of the aspartate residues. The second aspartate residue then donates a proton to the leaving group, facilitating the release of the cleaved peptide fragment.
Substrate Specificity
Serine proteases and aspartic proteases exhibit different substrate specificities, which are determined by the structure of their active sites.
Serine proteases typically cleave peptides with basic residues at the P1 position (the position immediately after the scissile bond). Aspartic proteases, on the other hand, prefer substrates with acidic residues at the P1 position.
The substrate specificity of these enzymes is crucial for their physiological functions, as it allows them to target specific proteins for hydrolysis.
Query Resolution
What are the key structural differences between serine proteases and aspartic proteases?
Serine proteases possess a catalytic triad consisting of serine, histidine, and aspartate, while aspartic proteases have a catalytic dyad of two aspartic acid residues. These structural variations contribute to their distinct catalytic mechanisms.
How do serine proteases and aspartic proteases differ in their substrate specificities?
Serine proteases typically cleave peptides after basic amino acids, while aspartic proteases prefer acidic amino acids. This difference in substrate specificity arises from the distinct interactions between the enzyme active sites and the substrate.
What are some examples of physiological functions performed by serine proteases and aspartic proteases?
Serine proteases participate in digestion, blood coagulation, and immune responses, while aspartic proteases are involved in protein degradation and apoptosis.