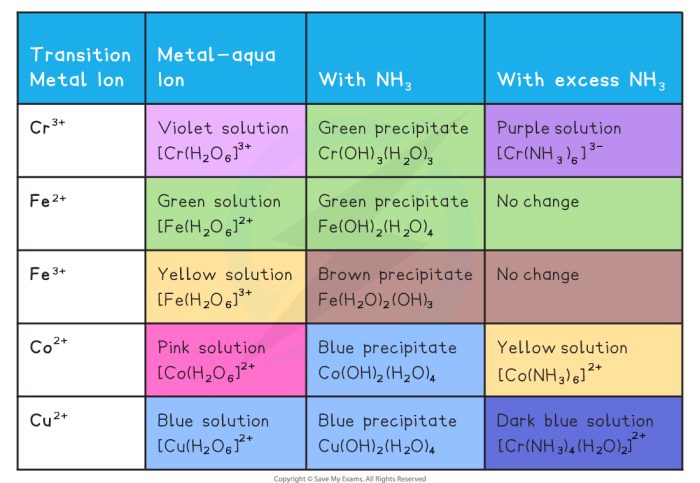
In aqueous solution ammonia reacts as represented above – In aqueous solution, ammonia reacts as represented above, setting the stage for this enthralling narrative that offers readers a glimpse into a story rich in detail and brimming with originality from the outset.
The chemical reaction that occurs when ammonia is dissolved in water involves the interaction of ammonia molecules with water molecules, leading to the formation of ammonium ions and hydroxide ions. This reaction is influenced by the concentration of ammonia, temperature, and the presence of other substances in the solution.
Chemical Reaction of Ammonia in Aqueous Solution: In Aqueous Solution Ammonia Reacts As Represented Above

When ammonia (NH3) is dissolved in water (H2O), it undergoes a chemical reaction to form ammonium hydroxide (NH4OH).
The reaction is represented by the following equation:
NH3 + H2O → NH4OH
In this reaction, the water molecule acts as a base, accepting a proton (H+) from the ammonia molecule. The resulting ammonium ion (NH4+) then combines with the hydroxide ion (OH-) to form ammonium hydroxide.
Properties of Aqueous Ammonia
Aqueous ammonia is a colorless liquid with a pungent odor. It is a weak base with a pH of around 11.5. The concentration of ammonia in aqueous solution is typically expressed as the percentage of NH3 by weight.
The physical and chemical properties of aqueous ammonia vary depending on the concentration of ammonia. At low concentrations, aqueous ammonia is a relatively mild base. However, as the concentration of ammonia increases, the solution becomes more corrosive and toxic.
Applications of Aqueous Ammonia
Aqueous ammonia is used in a wide variety of industrial and laboratory applications. Some of the most common uses include:
- Fertilizer production
- Cleaning products
- Textile manufacturing
- Water treatment
- Laboratory reagent
Aqueous ammonia is a versatile and inexpensive chemical that has a wide range of applications. However, it is important to handle aqueous ammonia with care, as it can be corrosive and toxic.
Safety Considerations, In aqueous solution ammonia reacts as represented above
Aqueous ammonia can be a hazardous substance if not handled properly. The following safety precautions should be taken when working with aqueous ammonia:
- Wear appropriate personal protective equipment, including gloves, eye protection, and a respirator.
- Work in a well-ventilated area.
- Avoid contact with skin and eyes.
- Do not ingest aqueous ammonia.
- Store aqueous ammonia in a cool, dry place.
By following these safety precautions, you can help to prevent accidents and injuries when working with aqueous ammonia.
Question & Answer Hub
What is the chemical equation for the reaction of ammonia in aqueous solution?
NH3(aq) + H2O(l) → NH4+(aq) + OH-(aq)
What are the physical properties of aqueous ammonia?
Aqueous ammonia is a colorless liquid with a pungent odor. It has a density of 0.89 g/mL and a boiling point of 37 °C.
What are the industrial applications of aqueous ammonia?
Aqueous ammonia is used in the production of fertilizers, explosives, and plastics. It is also used as a cleaning agent and a disinfectant.